The following events were observed the day of the game (01/18/2015):
- It was revealed on 02/19/2015 that the Indianapolis Colts (the visiting team) had reported concerns about rumors of low football pressure at previous Patriots games to the NFL in the week before the game.
- The game was held in the open Gillette stadium in Foxborough Massachusetts.
- Two hours before the game, the 12 Patriots footballs were properly inflated and tested by officials in a locker room at 78 °F. They were then impounded by the officials until just before the game as required by the rules.
- Just before kickoff, the Patriots balls were delivered to the ball boys to be used during the game. The temperature at the time was 52 °F.
- One of the Patriot balls was intercepted by a Colts player, who threw it to the Colts equipment manager. The equipment manager noticed it was underinflated, and notified Colts coaches. The coaches notified the league officials.
- At the beginning of halftime, the footballs were impounded again as the rules require. The temperature at the time was 39 °F and it was raining.
- During halftime, the officials took all of the Patriots footballs out of the game and replaced them with the back-up balls, which were tested and inflated to proper pressure.
- At the beginning of the second half, a Patriot snap of the ball was delayed while a different ball was brought in.
- After the game, the officials reported that they had found 11 of the 12 Patriot footballs were 2 psi below minimum pressure required by rule.
The following events occurred after the day of the game:
- When NFL officials tested the footballs indoors the next day, the pressure had increased to nearly the normal pressure. This baffled the officials.
- On 02/01/2016, two weeks after this game, the NFL announced that they had a person they were investigating. They said they were looking into what a ball handler was doing during the first half.
- On 02/01/2016, Tom Brady and the New England Patriots won Super Bowl XLIX.
- Two days after that, the NFL announced that only one of the 12 footballs was significantly below the minimum pressure. The other footballs were 0.1 psi to 0.2 psi below the minimum pressure.
- On 02/15/2015, the NFL revealed that investigation showed that an NFL employee had been stealing and selling NFL game memorabilia, including game footballs.
- On 05/06/2015, the NFL revealed that an independent investigation by Tedd Wells showed that two NFL employees had "more probably than not" tampered with the footballs, and that Tom Brady probably knew it was being done.
- On 05/11/2015, the NFL suspended Tom Brady for 4 games and penalized the Patriots with a fine of $1 million and two draft picks. They also suspended locker room attendant Jim McNally and equipment assistant John Jastremski. McNally had taken all of the primary Patriot game balls into a restroom for one minute and 40 seconds just before the game. The report says "the condition of the footballs was the result of deliberate actions by employees of the Patriots."
- On 05/14/2015, Tom Brady appealed his suspension.
- On 05/15/2015, the NFL announced that Commissioner Roger Goodell would himself hear Tom Brady's appeal.
- On 05/18/2015, Robert Kraft, owner of the Patriots, announced he will not appeal the NFL ruling, setting science back to the days of Aristotle.
- On 05/19/2015, the author of this page discovered that it would cost over $800 to buy a pressure meter that can accurately produce the reported readings. The meter is a 4-inch cube. So what meter was the NFL using? Cheap sports or automotive meters are not accurate enough to give the necessary 3 digits of accuracy. But it looked on TV like the officials had small pocket meters.
- On 05/22/2015, the NFL refused the player's union request that Commissioner Roger Goodell should recuse himself in Tom Brady's appeal.
- On 07/29/2015, the NFL released a new set of rules for checking football pressures. They are not designed to detect and correct pressure changes caused by temperature changes.
- On 07/28/2015, NFL Commissioner Roger Goodell denied Tom Brady's appeal.
- On 07/29/2015, the players' union sued the NFL in federal court in behalf of Tom Brady.
- On 05/18/2015, Robert Kraft, owner of the Patriots, announced that he made a mistake when he decided not to appeal the NFL ruling.
- On 07/30/2015, Federal Judge Richard Berman starts the deflategate hearings.
- On 09/03/2015, Federal Judge Richard Berman ruled that Goodell violated player rights and used tenuous evidence. He vacated all penalties.
- On 09/04/2015, Roger Goodell appealed Judge Berman's ruling.
- On 12/18/2015, New York Law professor Robert Blecker filed an amicus curiae (friend of the court) brief saying that the NFL's investigation of Brady's alleged ball-tampering was biased, unfair, partial, and fraudulent. But the brief also contains errors in fact.
- On 04/25/2016, the appellate court ruled in favor of Goodell. Stupidly, the 4-game suspension of Brady was restored.
- On 07/13/2016, the 2nd US Circuit Court of Appeals denied the appeal of the NFL Players' Association, deciding in favor of Goodell. Stupidly, the 4-game suspension of Brady remains. Note that NONE of these court decisions considered the scientific facts. They ruled on only whether or not Goodell has the power to decide.
- Stupidly, Goodell and the NFL made Tom Brady serve the 09/03/2016 to 10/03/2016, 4-game suspension for something that science has proved did not happen. Brainless "common sense" wins, while science and justice lose.
- On 02/05/2017, Tom Brady went on to win Super Bowl LI in spite of Goodell's bad science and wrong decisions. He was obviously not affected very much by the suspension.
The rules require the ball to weigh between 14 and 15 ounces, and that it must be inflated to between 12.5 and 13.5 pounds per square inch (psi).
No. Once the footballs are inflated to between 12.5 and 13.5 pounds per square inch (psi), the rules wrongly expect that pressure will stay the same during the entire game.
NO! 13 pounds is bowling-ball weight. A flying 13 lb. ball could kill someone.
The ball weighs between 14 and 15 ounces (16 ounces is one pound).
The air pressure in the ball is 13 pounds per square inch (psi).
And why is a "foot"-ball defined to be only 11 inches long?
It wouldn't be hard for someone to stick a ball needle into one football and deflate it somewhat without being seen. But it would be very hard for someone to deflate all of them to exactly the same lower pressure. That takes time, skill, and a pressure gage.
Very little, since the footballs are impounded in the officials' locker room during the times the game is not in actual play. During the times the game is in play, the footballs are in plain view, where any TV camera, security camera, or cell phone camera could record any tampering. Also, someone would have had to have tampered with the footballs again while they were impounded after the game, to raise the pressure for the examination by the officials.
McNally took all 12 of the primary Patriot footballs into a restroom just before the game, in violation of the rules. But one minute and 40 seconds gives him only 8 seconds per football to lower it by the same psi value for each ball. This is not enough time to do what the NFL claims.
They would need actual proof that someone intentionally committed an act to deflate the footballs used in the game.
He wanted to keep it as a souvenir of intercepting a pass in a playoff game. He said that he had not noticed anything wrong with that ball.
He didn't get to keep it, because the officials impounded it.
One of the special balls marked and used for kicking was still in the game after the kickoff. It was supposed to be changed out before a scrimmage play, but this had not been done.
Just like all other objects, footballs have to obey the laws of physics, including the gas laws. Changes in temperature, evaporating rain, and barometric pressure affect the pressure inside the football.
HeadSmart Labs found that similar weather changes caused an average 1.8 psi drop in football pressure.
He stated that the only way for the pressure to drop was for someone to let air out of the football. But this does not agree with the gas laws.
Some of the NFL officials also deny that it can happen.
Nye has been caught using bad science before. He has done this when siding with a liberal view (e.g. global warming caused by man, dangers from fracking, and hereditary homosexuality).
There are two requirements:
- The temperature must be in units of absolute temperature.
Absolute temperature is measured in Kelvins or Rankines. An absolute temperature scale has as its zero point absolute zero - the coldest anything can ever get.
Temperatures measured in degrees Celsius or degrees Fahrenheit are based on arbitrarily chosen zero points. They must be converted to one of the absolute scales to be used with the gas laws. Either scale will do, as long as all temperatures are converted to the same scale.
- The pressure must be measured in absolute pressure, not gage pressure.
Absolute pressure is the pressure of some gas referenced to a vacuum.
Gage pressure is referenced to the ambient air pressure.
Gage pressure must be converted to absolute pressure to be used with the gas laws.
Footballs (and all inflatable sports balls) must obey the gas laws.
Note that temperatures in Kelvins or temperatures in Rankines can be used to calculate the temperature ratio. But with temperatures in Fahrenheit, it is easier to use Rankines.
In the following tables:
ini = initial
fin = final
abs = absolute
P = pressure (psi)
T = temperature
A = atmospheric pressure (psi)
B = barometric pressure (inHg)
Z = Volume expansion factor of the football.
| CONVERSION | CALCULATE VALUE | UNITS |
|---|---|---|
| Convert temperature to absolute | T (R) = T (°F) + 460 [for more accuracy: 459.67] | °F, R (Rankine) |
| Convert temperature from absolute | T (°F) = T (R) - 460 [for more accuracy: 459.67] | °F, R (Rankine) |
| Convert gage pressure to absolute | P (abs psi) = P (gage psi) + A [if A is not known, use 14.7 psi (abs)] | psi (gage), psi (abs) |
| Convert absolute pressure to gage pressure | P (gage psi) = P (abs psi) − A [if A is not known, use 14.7 psi (abs)] | psi (abs), psi (gage) |
| Convert of barometric pressure (inHg) to psi | A (abs psi) = B (abs inHg) × 0.492 | inHg (abs), psi (abs) |
| PHYSICAL EFFECT | VARIES WITH | CALCULATE EFFECT | UNITS |
|---|---|---|---|
| Change in temperature | Proportional Direct | P fin abs = (P ini abs) × (T fin abs) / (T ini abs) | psi, in Hg, °F |
| Change in temperature | Proportional Direct | P fin gage = (P ini gage + A) × (T fin abs) / (T ini abs) - A | psi, in Hg, °F |
| Wind Chill effect on temperature | Lowers Temperature | Aim an IR no contact thermometer at the football. | °F, mph, (rh)% |
| Change in barometric pressure | Proportional Direct | P fin abs = P ini abs × (A fin abs / A ini abs) × Z | psi, Nt |
| Humidity effect of temperature | Exponential Additive | Since condensation is complicated, it will not be shown here. |
The following are the physical reasons why these affect the pressure:
- The temperature of the air in the football determines how energetic the gas molecules inside the ball are. How energetic the molecules are determines how much they push against the envelope of the ball.
- Wind Chill in this case is the effect of the wind blowing on a wet football, cooling the ball by evaporative cooling. This lowers the temperature of the air inside the football.
- Because the envelope of the ball is not rigid, the amount the barometric pressure presses on the outside of the ball changes the pressure inside the ball. This effect is usually minute.
- Humidity of the air inside the ball affects the pressure in the ball if it condenses out of the air, forming water in the ball. This lowers the pressure inside the ball, because there are fewer molecules pushing against the envelope of the ball.
The last two are usually negligible.
The NFL officials reported they had the following readings:
- All footballs checked out as having the proper range of 12.5 psi to 13.5 psi when they were inflated.
- At halftime, the Patriots' footballs had readings of about 10.5 psi.
- The following day, the same footballs measured about 12.3 psi.
The NFL officials and the National Weather Service reported the following temperatures:
- The locker room where the Patriots' footballs were inflated was 78°F.
- Kickoff field temperature was 52°F.
- At halftime the field temperature was 39°F and the wind chill was 31°F.
Here are several possible scenarios, depending on original pressures and test room temperature:
All of the lines in the following tables are calculated from the following information using the gas laws:
- The reported pressures and temperatures above.
- Selected possible initial football pressures.
- Several possible temperatures in the room the next day.
Patriots' footballs:
- Case A: initial pressure was 12.5 psi; next day temperature was 68°F.
- Case B: Initial pressure was 13.0 psi; next day temperature was 68°F.
- Case C: Initial pressure was 13.5 psi; next day temperature was 68°F.
- Case D: Initial pressure was 12.5 psi; next day temperature was 72°F.
- Case E: Initial pressure was 13.0 psi; next day temperature was 65°F.
- Case F: Initial pressure was 13.0 psi; cheated down to 10.5 psi at kickoff; next day temperature was 68°F.
Colts' footballs:
- Case G: Initial pressure was 13.0 psi; next day temperature was 65°F.
- Case H: Initial pressure was 13.5 psi; next day temperature was 65°F.
What Goodell demands:
- Case I: Initial pressure was 13.0 psi; next day temperature was 68°F.
| C A S E |
Inflation Time | Kickoff Time | Halftime (no wind chill) | Halftime (with wind chill) | Test Next Day | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Press | Temp | temp chg ratio |
Press | Temp | temp chg ratio |
Press | WC Temp | temp chg ratio |
WC Press | Temp | temp chg ratio |
Press | |||||||||||||
| val °F |
abs R |
gage psi |
abs psi |
val °F |
abs R |
abs psi |
gage psi |
val °F |
abs R |
abs psi |
gage psi |
val °F |
abs R |
abs psi |
gage psi |
val °F |
abs R |
abs psi |
gage psi |
|||||||
| A | 78 | 538 | 12.5 | 27.2 | 52 | 512 | .952 | 25.9 | 11.2 | 39 | 499 | .928 | 25.2 | 10.5 | 31 | 491 | .913 | 24.8 | 10.1 | 68 | 528 | .981 | 26.7 | 12.0 | ||
| B | 78 | 538 | 13.0 | 27.7 | 52 | 512 | .952 | 26.4 | 11.7 | 39 | 499 | .928 | 25.7 | 11.0 | 31 | 491 | .913 | 25.3 | 10.6 | 68 | 528 | .981 | 27.2 | 12.5 | ||
| C | 78 | 538 | 13.5 | 28.2 | 52 | 512 | .952 | 26.8 | 12.1 | 39 | 499 | .928 | 26.2 | 11.5 | 31 | 491 | .913 | 25.7 | 11.0 | 68 | 528 | .981 | 27.7 | 13.0 | ||
| D | 78 | 538 | 12.5 | 27.2 | 52 | 512 | .952 | 25.9 | 11.2 | 39 | 499 | .928 | 25.2 | 10.5 | 31 | 491 | .913 | 24,8 | 10.1 | 72 | 532 | .981 | 26.9 | 12.2 | ||
| E | 78 | 538 | 13.0 | 27.7 | 52 | 512 | .952 | 26.4 | 11.7 | 39 | 499 | .928 | 25.7 | 11.0 | 31 | 491 | .913 | 25.3 | 10.6 | 65 | 525 | .976 | 27.0 | 12.3 | ||
| F | 78 | 538 | 13.0 | 27.7 | 52 | 512 | .952 | 25.2 | 10.5 | 39 | 499 | .928 | 24.6 | 9.9 | 31 | 491 | .913 | 24.2 | 9.5 | 68 | 528 | .981 | 26.0 | 11.3 | ||
| G | 52 | 512 | 13.0 | 27.7 | 52 | 512 | 1.00 | 27.7 | 13.0 | 39 | 499 | .974 | 27.0 | 12.3 | 31 | 491 | .959 | 26.6 | 11.9 | 65 | 525 | 1.025 | 28.4 | 13.7 | ||
| H | 52 | 512 | 13.5 | 28.2 | 52 | 512 | 1.00 | 28.2 | 13.5 | 39 | 499 | .974 | 27.5 | 12.8 | 31 | 491 | .959 | 27.0 | 12.3 | 65 | 525 | 1.025 | 28.9 | 14.2 | ||
| I | 78 | 538 | 13.0 | 27.7 | 52 | 512 | .952 | 27.7 | 13.0 | 39 | 499 | .928 | 27.7 | 13.0 | 31 | 491 | .913 | 27.7 | 13.0 | 68 | 528 | .981 | 27.7 | 13.0 | ||
| K E Y |
Ball Pressure Color |
Football Pressure Deviation From Rule |
Set Pressure Color |
Initial Football Set Pressure |
Temperature Color |
Test Area Temperature |
||
|---|---|---|---|---|---|---|---|---|
| P < 10.5 psi (> 2 psi low) | 12.5 psi | 65 °F | ||||||
| 10.5 psi ≤ P < 11.5 psi (1 to 2 psi low) | 13.0 psi | 68 °F | ||||||
| 11.5 psi ≤ P < 12.5 psi (up to 1 psi low) | 13.5 psi | 72 °F | ||||||
| Pressure is within NFL rules | Team Identification | |||||||
| P > 13.5 psi (high) | Patriots | Colts | ||||||
| If air was let out of footballs | Goodell Belief | Goodell "Fizzix" | ||||||
Note that the NFL didn't test any Colts' footballs the next day, so they didn't notice the overinflation.
Temperature Change Ratio is the multiplying factor applied to the temperature when the footballs were inflated or adjusted.
Note that in case F, where air is deliberately let out of the footballs, the halftime and next day readings are much lower than the readings the officials obtained. Thus, NO air was deliberately let out of the footballs.
Absolute Zero is the coldest anything can get. It is a total absence of energy.
Absolute temperature is a temperature scale with Absolute Zero as the zero point on the scale. These are four temperature scales. Two are based on absolute temperature, and two are based on common weather phenomena:
SI is used to indicate metric (system Internationale) units.
EN is used to indicate English (American) units.
| SCALE | SYS | ° SIZE | ABSOLUTE 0 | SALT & ICE | H2O FREEZES | HOTTEST DAY | H2O BOILS | H2O SPAN ° |
|---|---|---|---|---|---|---|---|---|
| Rankine | EN | F ° | 0 | 459.67 | 491.67 | 559.67 | 671.67 | 180 |
| Kelvin | SI | C ° | 0 | 255.37 | 273.15 | 310.93 | 373.15 | 100 |
| Celsius | SI | C ° | -273.15 | -17.78 | 0 | 37.778 | 100 | 100 |
| Fahrenheit | EN | F ° | -459.67 | 0 | 32 | 100 | 212 | 180 |
Only the kelvin and rankine scales are based on the actual heat energy in the gas. The others have arbitrary zero points, so their values are not useful for figuring energy content.
The scales were developed as more was discovered about temperature:
- The Fahrenheit scale was developed first with the invention of the thermometer. It is based on weather.
- The Celsius scale was designed to use the freezing and boiling points of water. It was originally the metric system temperature.
- The Kelvin scale was developed when Absolute Zero was discovered. It uses the Celsius scale degree, and is the current SI unit.
- The Rankine scale uses Absolute Zero and the Fahrenheit degree.
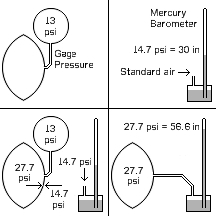 There are three ways to measure pressure:
There are three ways to measure pressure:
- Absolute pressure measures the difference between the measured pressure and a perfect vacuum. A column of mercury with a perfect vacuum over it is used to measure barometric pressure (image, upper right) and absolute pressure (image, lower right).
- Aneroid pressure measures the difference between the measured pressure and the standard air pressure (14.7 psi). An aneroid pressure meter has a sealed bellows in it that contains air at standard pressure, which is compared with the pressure connected to the meter (image, upper left).
- Gage pressure measures the difference between the measured pressure and the ambient air pressure (barometric pressure). A gage pressure meter has a bellows in it open to the air on one side, which is compared with the pressure connected to the meter (image, lower left).
Only absolute pressure measures all of the energy in the gas. The others leave out anything below standard air pressure.
Note that when using a mercury barometer to measure pressure in something as small as a football, the diameter of the measuring tube must be small. Otherwise, enough air to get the pressure reading is taken from the football, which also somewhat deflates the football. This produces a lower reading than the original absolute pressure in the football.
Only absolute pressure is related to the energy in the gas that is creating the pressure. The others have arbitrary zero points based on the pressure of the air, so they do not have all of the pressure in the reading.
They were all developed for different metric and English uses.
- The atmosphere and the bar are close to normal air pressure.
- The torr and inHg are based on the mercury barometer.
- The pascal and psi are basic units derived from the measurement systems.
SI is used to indicate metric (system Internationale) units.
EN is used to indicate English (American) units.
| PRESSURE SCALE - ABSOLUTE | SYS | VACUUM | STANDARD AIR | PURPOSE OF UNIT | DERIVATION OF UNIT | |
|---|---|---|---|---|---|---|
| A B S O L U T E |
Atmosphere (Atm) | EN | 0 atm | 1 atm | Pressure of Gases | Standard air pressure |
| Pascal (Pa) | SI | 0 Pa | 101325 Pa | SI Metric Pressure Unit | 1 Newton / Square Meter | |
| Millibar (mbar) = 1 Hectopascal (HPa) | SI | 0 mbar | 1013.25 mbar | Barometric Pressure | 100 Pa, .001 bar | |
| Kilopascal (KPa) | SI | 0 KPa | 101.325 KPa | Metric Gas Pressure | 1000 Pa, .01 bar | |
| Bar | SI | 0 bar | 1.01325 bar | Barometric pressure | 100000 Pa | |
| Torr | SI | 0 torr | 760 torr | Barometric Pressure | millimeters of Hg column | |
| Inches Hg | EN | 0 inHg | 29.92 inHg | Barometric Pressure | Inches of Hg column | |
| Pounds Per Square Inch (psi) | EN | 0 psi | 14.69595 psi | American Gas Pressure | Pound per Square Inch | |
| PRESSURE SCALE - ANEROID & GAGE | SYS | VACUUM | STANDARD AIR | PURPOSE OF UNIT | DERIVATION OF UNIT | |
| G A G E |
Pascal (Pa) | SI | -101325 Pa | 0 Pa | SI Metric Pressure Unit | 1 Newton / Square Meter |
| Kilopascal (KPa) | SI | -101.325 KPa | 0 KPa | Metric Gas Pressure | 1000 Pa, .01 bar | |
| Bar | SI | -1.01325 bar | 0 bar | Pressure of Gases | 100000 Pa | |
| Pounds Per Square Inch (psi) | EN | -14.69595 psi | 0 psi | American Gas Pressure | Pound per Square Inch |
Use the following procedure:
Note that this procedure assumes that expansion does not occur along the long dimension.
Note that this factor probably will not be needed. It is small.
Materials needed:
- One regulation football
- One sports inflation pump with psi gage, check valve, and dump valve.
- One tape measure (either inches or centimeters).
Procedure:
- Pump up the football to exactly 15.0 psi.
- Measure the football circumference around the largest diameter perpendicular to the long dimension.
- Write down the gage pressure and the circumference. Label them P ini and C ini
- Divide C ini by 3.5. Then square the result. Write it down as V ini.
- Change the football pressure to exactly 13.5 psi.
- Measure the football circumference around the largest diameter perpendicular to the long dimension.
- Write down the gage pressure and the circumference. Label them P fin and C fin
- Divide C fin by 3.5. Then square the result. Write it down as V fin.
- Subtract V fin from V ini. Then divide the result by V ini. Write down the result of this to 3 significant digits as V dif
- Subtract P fin from P ini. Then divide the result by P ini.
Write down the result of this to 3 significant digits as P dif
Note: If your values were the same as the instructed pressures, P div should be 0.100 - Divide V dif by P dif to get Z.
This is still being researched by the page author.
 The best way to find it out is to use an infrared no-contact thermometer to measure the
actual temperature of the football at the time it is needed. The page author's unit is shown
at right.
The best way to find it out is to use an infrared no-contact thermometer to measure the
actual temperature of the football at the time it is needed. The page author's unit is shown
at right.
For now, the wind chill chart for human beings is close enough if no reading is available.
There are several different kinds of wind chill:
- The effect of moving air passing over a dry surface, transferring heat to or from the
surface, brings its temperature closer to the air temperature by bringing the air temperature
closer to the temperature of the surface. This is a heat exchanger.
This would be the dry bulb temperature of a psychrometer if the ambient wind can contact the dry bulb.
Examples of this:
- Automobile radiator
- Outside fan on air conditioner
- Cooling fan on electric motor
- Wind blowing on a sealed jar of water
- Wind blowing on a dry football
- The effect of moving air passing over a wet surface, taking heat from the surface and from
the air, cools both by evaporative cooling. This cools both below the air temperature.
This is the modified wet bulb temperature of a psychrometer where, instead of air being mechanically forced across the wet bulb, the ambient wind moves the air across the wet bulb. Nobody seems to have a formula for this.
Examples of this:
- Wind blowing across a puddle
- A fan blowing on the wet surface of a swamp cooler
- A mist spray cooling fan
- Wind blowing on an open jar of water
- Wind blowing on a wet football
- The effect of moving air passing over a moist heated surface, transferring heat to or
from the surface to bring its temperature closer to, or lower than, the air temperature
through convection and evaporative cooling.
This is the wind chill in the wind chill tables the government publishes.
Examples of this:
- Wind blowing on a human body outdoors
- A fan used to cool a human body on a hot day
- Wind affecting an animal (a different calculation is needed for each specie)
- Power plant cooling tower (a different calculation is needed)
- Wind blowing across a warmer lake (a different calculation is needed)
The modified dry bulb temperature is the temperature of an ordinary thermometer, a dry surface measured with an infrared (IR) no-contact thermometer, or the dry bulb of a psychrometer that is exposed to the ambient wind, but not to sun or rain.
The reason for doing this is to find the temperature of a dry football, rather than calculating the humidity of the air.
This temperature can be taken several ways:
- Use an IR no-contact thermometer and a dry football in the wind, but not the sun.
- Use an IR no-contact thermometer and a dry surface in the wind, but not the sun.
- Use a thermometer with the bulb kept dry and in the wind, but not the sun.
- Use a sling psychrometer with the dry bulb in the wind, but not the sun.
The modified wet bulb temperature is the temperature of an ordinary thermometer with a wet tissue or cloth on the bulb, a wet surface measured with an infrared (IR) no-contact thermometer, or the wet bulb of a psychrometer that is exposed to the ambient wind, but not to sun or rain.
The reason for doing this is to find the temperature of a wet football, rather than calculating the humidity of the air.
This temperature can be taken several ways:
- Use an IR no-contact thermometer and a wet football in the wind, but not the sun.
- Use an IR no-contact thermometer and a wet surface in the wind, but not the sun.
- Use a thermometer with the bulb in a wet tissue in the wind, but not the sun.
- Use a sling psychrometer with the wet bulb in the wind, but not the sun.
Try this simple experiment.
Materials needed:
- One regulation NFL football
- One sports inflation pump with psi gage, check valve, and dump valve.
- One food freezer with room to hold a football.
- One infrared no-contact thermometer.
Procedure:
- Go into a warm room with the football, pump, and thermometer.
- Pump up the football to exactly 13 psi.*
- Aim the thermometer at the football and take the reading.
- Write down the gage pressure and football temperature.
- Put the football into the freezer.
- Wait 2 hours (to make sure the air in the football achieves uniform temperature).
- Measure the pressure in the football.
- Aim the thermometer at the football and take the reading.
- Write down the gage pressure and football temperature.
- Take the football back into the warm room.
- Wait 2 hours.
- Measure the pressure in the football.
- Aim the thermometer at the football and take the reading.
- Write down the gage pressure and football temperature.
- Compare the measured pressures and temperatures. Construct a table like the one below.
(Prepare to duck, as your wife throws things at you because you took the roast out of the freezer to make room for the football.)
* If a regulation football will not fit in the freezer, use a junior football and a pressure of 9 psi.
Sample data:
| Experiment Data | Measurement 1 | Measurement 2 | Measurement 3 | |
|---|---|---|---|---|
| Case | Warm Room | Freezer | Warm Room | |
| Measured Temperature F (°F) | 80 °F | 0 °F | 80 °F | |
| Measured Gage Pressure G (psi) | G1: 13.0 psi | G2: 9.0 psi | G3: 12.9 psi | |
| Find abs temperature T (Rankine) by adding 460 to F | T1: 540 R | T2: 460 R | T3: 540 R | |
| Find abs pressure A (psi) by adding 14.7 psi to gage psi | A1: 27.7 psi | A2: 23.7 psi | A3: 27.6 psi | |
| Calculation of new absolute pressure B | A1 X T2 / T1 = B2 | A2 X T3 / T2 = B3 | ||
| Calculate new pressure B from temperature change | 27.7 psi X 460 R / 540 R = 23.596 | 23.7 psi X 540 R / 460 R = 27.821 | ||
| Calculated absolute pressure B (rounded) | B2: 23.6 psi | B3: 27.8 psi | ||
| Find gage psi P by subtracting 14.7 psi from abs psi | P2: 8.9 psi | P3: 13.1 psi | ||
| Calculated | Observed | Calculated | Observed | |
| Compare Calculated P and Observed G pressures | P2: 8.9 psi | G2: 9.0 psi | P3: 13.1 psi | G3: 12.9 psi |
No air left the footballs, and no air re-entered the footballs. Try the above experiment to find this out for yourself.
Note that the act of measuring can let a tiny amount of air out of the ball.
It is a fallacy that the only thing that affects the pressure inside a football is the number of air molecules inside the football.
The speed of air molecules depends on the absolute temperature of the air.
- When the temperature is low, the molecules move at lower speeds.
- When the temperature is high, the molecules move at higher speeds.
The pressure of the air against the inside of the football is a result of the air molecules hitting each other and hitting the inside of the envelope of the football. So changing the temperature changes the speed of the molecules.
- The absolute temperature rises
- The air molecules move faster
- The air molecules hit each other harder
- The air molecules hit the inside of the football harder
- So the pressure in the football is higher.
Thus, the following happened during the game and the next day:
- Initially, the temperature was high (538 R), and the air molecules were moving fast enough that the footballs had full pressure.
- At halftime, the temperature was low (491 R), and the air molecules were moving at lower speeds, so the football pressure was low.
- When the footballs were examined the next day, the temperature was higher again (around 528 R), so the air molecules were moving fast enough again that the footballs had nearly full pressure.
The temperature changed the pressure without any air leaving or entering the footballs.
It affects only inflatable pressurized balls that are used outdoors:
- Baseball, softball, cricket, tennis, croquet, handball, bowling, and lacrosse do not use pressurized balls, so this effect does not occur in these games.
- When football, basketball, volleyball, soccer, and other games with pressurized balls are played indoors, this effect does not occur because the temperature does not change.
- When football, basketball, volleyball, soccer, and other games with pressurized balls are played outdoors, this effect can occur when the weather changes.
Yes: Temperature affects automobile tire pressure.
Cold weather reduces the pressure in your tires, even though no air leaks out. You must add more air
When the weather warms up, the pressure is then too high.
Calculation shows a 33.0 psi tire pressure at 80°F would reduce to 29.4 psi at 39°F. Only a driver in a pressure-sensitive vehicle would notice this change.
It probably has been noticed before, but nobody was ever claiming it was done deliberately before.
Several things combined to make it noticeable in this particular case:
- Pressure gages accurate enough to detect the difference were used.
- The game was in an open stadium.
- The footballs were inflated and tested in a warm locker room on a cold day.
- The temperature dropped 13 °F during the first half.
- The rain caused a wind chill effect, lowering further the temperature in the footballs.
- It was windy.
- There was no sun.
- The footballs were kept in a place exposed to the weather.
- A Colts defensive man intercepted a pass and wanted to keep the ball as a souvenir.
- The Colt's equipment manager noticed the souvenir ball felt soft, starting the investigation.
- The officials were wary, because another team had earlier complained about underinflated footballs when playing the Patriots in Foxborough.
- Usually a single underinflated ball is thought to be a defective football. But this time, the number of underinflated footballs was too large to be a single failure.
- A reporter for an Indianapolis (home of the Colts) media outlet broke the story in an attempt to have the game outcome nullified.
This effect was never noticed before by players or officials. The following factors probably kept it from being noticed at any time before this game was played:
- Most pressure gages aren't accurate enough to detect the difference.
- The game was in a domed stadium, not open to the weather.
- The game was played in the southern US.
- Before 1967, they didn't play in the winter.
- The footballs were inflated and tested on the field.
- The weather was warm.
- The temperature didn't change much during the game.
- It wasn't raining.
- It wasn't windy.
- There was no large change in the weather.
- The game wasn't in Massachusetts, where temperatures tend to vary more in the winter.
- The footballs were kept in a place sheltered from the weather.
- The coach had a personal heater near the where footballs were kept.
- The sun shining on the football at times counteracted dropping temperatures.
- Nobody noticed anything unusual about any of the footballs.
- The officials were not tipped off to look for anything unusual.
- Only one ball seemed underinflated, so it was thought to be just a defective football.
- Nobody's grudge against the other team was strong enough to make someone think of cheating.
- The officials thought the other team was falsely complaining.
They made mistakes:
- Some of them without experience in gases believe the common-sense argument that the pressure can't change because no air enters or leaves the football.
- Many of these scientists didn't use absolute pressure or absolute temperature.
The page author also did this the first time he calculated it. That 14.7 psi of air pressure is easy to forget. But the physics book reminded him.
- Many of them did not know of the entire temperature range of 78°F to 39°F. Many of them had 52°F as either the beginning temperature or the ending temperature.
- The scientist calculating the change sees a 7.25% change as not significant.
The legal NFL football pressure range for football pressure is only ±1.8 % (absolute pressure) or ±3.8 % (gage pressure).
The legal NFL football pressure range is stricter than most scientific measurements.
Two weeks after this game, the NFL announced that they had a person they were investigating. They said they were looking into what a ball handler was doing during the first half. He had taken several balls into a restroom, one by one. But he had just been drying them there.
Two days later, they announced that all but one of the footballs were 0.1 to 0.2 psi below the lower inflation limit of 12.5 psi.
The problem is that NFL officials are totally baffled by what happened.
They still don't seem to believe that the air pressure in a football can change without air being added to or taken out of the football. They should try the freezer experiment above.
Officials with their heads in the sand believe that the weather can't change the pressure in a football, so they told investigators to look for who was altering the air pressure in the footballs. They wrongly did not ask for any scientific review.
No.
They believe they have proof that the footballs were deflated from the pressure readings they got, because they have the false belief that temperature changes do not change the pressure inside a football.
Using the actual temperatures, the laws of physics predicted almost perfectly the pressures the officials measured on the field at halftime and indoors the next day, but this is true only if nobody let any air out of the footballs.
The laws of physics predicted this too. See the football pressure table above.
- The Colts' footballs were inflated on the field at a temperature near 52°F.
- The Patriots' footballs were inflated in a locker room at 78°F.
- The Patriots' footballs lost 1.3 psi just from being carried out onto the field where the temperature was 52°F and remaining there a while. Thus, those footballs were probably already violating the rule before the game started.
- If the NFL had checked the Colts' footballs then next day, they would have found them overinflated.
There are several other factors that don't quite measure up.
- Most available pressure gages don't have the accuracy needed to accurately get the
observed readings.
Note that the presence of 3 digits on the display does not mean that all three digits are always significant.
Note that the specification expressed as ±2 psi can express itself one or more of these three possible ways:
- Proportional accuracy can apply a constant percent increase or decrease to all readings (with 40 psi full scale, a ±5.0 % error in the reading).
- Offset accuracy can add or subtract a constant value up to 2 psi to all readings.
- Successive accuracy readings can vary by ±2 psi due to the sample-collection device having friction to overcome.
Note that the specification expressed as ± 5% can express itself one or more of these three possible ways:
- Proportional accuracy can apply a constant percent increase or decrease to all readings (with 40 psi full scale, a ±5.0 % change is an error of ±2 psi).
- Offset accuracy can add or subtract a constant value up to 2 psi (5% of 40 psi) to all readings.
- Successive accuracy readings can vary by ±2 psi (5% of 40 psi) due to the sample-collection device having friction to overcome.
Note that the specification usually does not tell you how much of each kind of error is in the instrument. It tells only the maximum error.
Specification Full Scale Half Scale NFL Football at Exactly 13.0 psi Accuracy Method Reading Accuracy Reading Accuracy Next Read Min Max Useful? Needed Spec ±2 psi F S Proportional 40 psi ±2 psi 20 psi ±1 psi same 12.3 psi 13.7 psi NO ±0.3 psi ±2 psi Offset 40 psi ±2 psi 20 psi ±2 psi same 11.0 psi 15.0 psi NO ±0.1 psi ±2 psi Successive 40 psi ±2 psi 20 psi ±2 psi ±2 psi 11.0 psi 15.0 psi NO ±0.1 psi ±2 psi All 3 Equally 40 psi ±2 psi 20 psi ±1.7 psi ±0.7 psi 11.0 psi 15.0 psi NO ±0.1 psi ±5% Proportional 40 psi ±2 psi 20 psi ±1 psi same 12.3 psi 13.7 psi NO ±0.7% ±5% F S Offset 40 psi ±2 psi 20 psi ±2 psi same 11.0 psi 15.0 psi NO ±0.25% ±5% F S Successive 40 psi ±2 psi 20 psi ±2 psi ±2 psi 11.0 psi 15.0 psi NO ±0.25% ±5% F S All 3 Equally 40 psi ±2 psi 20 psi ±1.7 psi ±0.7 psi 11.6 psi 14.4 psi NO ±0.25% The page author tried to buy a pressure gage with the accuracy needed for the reported pressures. He found the following, where either the price or the lack of accuracy made it impossible for him to do the above experiment with an accuracy of 3 significant digits:
The Legal Pressure Reading Range is the ball pressure with the benefit of any doubt for accuracy errors.
Specifications Possible Readings at Exactly 13,0 psi Legal Pressure Reading Range Unit Price Full Scale Accuracy Low at 13.0 psi High at 13.0 psi Useful? Lowest Legal Highest Legal Needed Accuracy ? 30 psi ±0.05 psi 12.95 psi 13.05 psi Yes 12.45 psi 13.55 psi Wilson NCAA sport $17 30 psi ±2 psi 11.0 psi 15.0 psi NO 10.5 psi 15.5 psi Champion tire $16 60 psi ±2 psi 11.0 psi 15.0 psi NO 10.5 psi 15.5 psi Pep Boys tire $16 60 psi ±2 psi 11.0 psi 15.0 psi NO 10.5 psi 15.5 psi Markworks sport $46 20 psi ±0.2 psi 12.8 psi 13.2 psi Marginal 12.3 psi 13.7 psi Digi-Gage sport $35 30 psi ±0.4 psi 12.6 psi 13.4 psi NO 12.1 psi 13.9 psi Dwyer sport $13 50 psi ±1 psi 12.0 psi 14.0 psi NO 11.5 psi 14.5 psi Omega lab $940 500 psi ±0.1% full 12.5 psi 13.5 psi NO 12.0 psi 14.0 psi Dwyer lab $1100 1000 psi ±0.05% full 12.5 psi 13.5 psi NO 12.0 psi 14.0 psi Setra lab $1500 100 psi abs
(85.3 psi ga)±0.05 psi 27.65 psi
(12.95 psi)27.75 psi
(13.05 psi)Yes 27.15 psi
(12.45 psi)28.25 psi
(13.55 psi) - A Wilson "Official NFL Football" found in a sporting goods store had printed
on it an allowed pressure range of 11 psi to 13 psi.
This means that pressures allowed by the NFL violate the specifications of the ball made for this purpose.
- The NFL requirement of 13.0 psi ± 0.5 psi is a range that is tighter than the range
of tolerance of most scientific instruments.
- This range (using absolute pressure) is 13 psi ±1.8 %.
- Even using gage pressure, this range is 13 psi ±3.8 %.
- Only the most expensive equipment has a tolerance range of better than ±5.0 % of the full scale reading.
- A cheap pressure gage with a range of 30 psi would have a ±5.0% range of ±1.5 psi (gage pressure).
- A cheap pressure gage with a range of 60 psi would have a ±5.0% range of ±3.0 psi (gage pressure).
- The fact that Commissioner Roger Goodell would not recuse himself from Tom Brady's appeal showed that he intended to use his authority as the boss to force the players to believe the erroneous common-sense belief that temperature can't affect football pressure and that their pressure measuring devices are accurate enough.
Tom Brady broke his cell phone. Roger Goodell claims it was done on purpose to keep the NFL from obtaining it. This is wrong for the following reasons:
- The NFL is not a government crime-investigation agency. It has no subpoena power. A recent court decision says an employer does not have the right to search an employee's personal cell phone, personal computer, or personal social media site. So Goodell had no right to demand Brady's cell phone.
- Goodell had no right to claim that Brady "wasn't cooperating" because his demand for the cell phone was unconstitutional.
- Breaking the phone does not destroy the files. The telephone service keeps the files.
Replacing a broken phone does not cause the loss of any saved emails or texts.
The page author's wife dropped her cell phone and it broke. The replacement phone had not lost any of the saved data.
- Brady was not under any compulsion to keep any emails or texts, because the phone was his personal phone. He could have deleted them at any time with no penalty.
One possibility is that Brady threw the phone as hard as he could when he found out that Goodell was accusing him of tampering with football pressure.
Judge Berman ruled that Roger Goodell violated several laws on arbitration by not properly notifying Brady and others, and by not allowing the accused employees access to the evidence and investigators.
Goodell appealed the Judge's decision.
Amazingly, the evidence of the football pressures did not enter into the decision. If the judge had properly investigated this, Goodell would have absolutely no case.
There were several errors:
- He claimed that the league released false pressure readings the night of the game. In fact, the science shows those readings were quite accurate (see above).
- He contended that the NFL released a misleading assertion that the Patriots balls were illegally deflated while all measured Colts balls were legal. This was actually true, but it was not deliberately done (see above).
- He contended that it mattered which gage the officials used. In fact, neither one has the needed accuracy to determine whether or not a violation occurred. Most gages of that size have an accuracy limit of ±1 psi or more. That makes them useless for this purpose (see above).
The problem is that the ruling says that Goodell has the legal right to impose the suspension, but it does not deal with the facts in the case. It let Goodell impose the penalty but did not cover whether or not the infraction really occurred.
So Goodell can reinstate the 4-game suspension. This wrongly punishes Tom Brady for something the evidence shows that he never did anything to cause.
Because Goodell still does not believe in the laws of physics, he wants to punish Brady for events that he believes Brady caused, but which were really caused by the weather.
The problem is that the ruling was about Goodell having the legal right to impose the suspension, but it again does not deal with the facts in the case.
NONE of these court decisions considered the scientific facts. They ruled on only whether or not Goodell has the power to decide.
The problem is that the old rule expects the pressure inside the football to remain the same during the entire game because no air enters or leaves the ball.
The rules do not specify anything about changes in temperature causing changes in the football pressure, because those who wrote the rules don't believe it can happen.
The old NFL rules require a pressure range tighter than the tolerances of most measuring equipment.
The measuring equipment the NFL uses is inadequate for detecting rules violations.
The problem is that the current rule still expects the pressure inside the football to remain the same during the entire game because no air enters or leaves the ball.
The rules still do not specify anything about changes in temperature causing changes in the football pressure, because those who wrote the rules still don't believe it can happen.
The NFL rules still require a pressure range tighter than the tolerances of most measuring equipment on the market.
The measuring equipment the NFL uses is inadequate for detecting rules violations.
The NFL must change its opinion on temperature affecting football pressure.
They believe in their "common sense" over real science:
- They believe in the common-sense belief that the number of molecules of air in the football defines its pressure. They can't see how changes in temperature can change the football pressure, since the amount of air in the ball remains the same.
- They see the air molecules as being like stationary stuffing in the football that does not change size.
But in this case, common sense is wrong.
- What they don't understand is that changes in temperature cause changes in football pressure by changing the speed of the air molecules, not the number of air molecules. The warmer a gas molecule is, the faster it moves. The faster a gas molecule travels, the more pressure it exerts, because it collides with everything with more force.
- Air molecules are constantly colliding with each other and with any containing surface. These collisions are what we call pressure. Gas molecules colliding with the diaphragm in the pressure meter produce the reading on the gage. Increasing the temperature causes those gas molecules to hit that diaphragm with more force, increasing the reading.
- The fact that they refuse to believe in the science of the gas laws indicates that they either believe that their authority can make their rules supersede science, or that common sense, rather than science, applies to footballs.
- They believe they can set any tolerance they want, without knowing the science that shows that their tolerances are way too tight for existing measuring equipment.
They must be changed to provide for the fact that air temperature and wind chill can change the pressure inside a football
- Each official needs the following knowledge:
- An understanding of the real laws of physics (the gas laws)
- An understanding of absolute vs gage pressure
- An understanding of absolute vs weather temperature
- An understanding of the necessary calculations (or a table)
- An understanding of pressure gage accuracy
- An understanding that the "common-sense" beliefs of Roger Goodell are wrong
- The following equipment is needed:
 An accurate enough pressure gage that does not lose air
An accurate enough pressure gage that does not lose air
- A sporting goods store pressure gage is not accurate enough.
- An automotive store pressure gage is not accurate enough.
It costs money to get the accuracy the NFL rules require.- An infrared no-contact thermometer gun
- The following rules must be applied:
- Inflate footballs on the sidelines, not in a locker room.
- Measure the temperature when and where the footballs are inflated.
- Any area where balls are impounded must be kept at field temperature, not at any indoor temperature.
- Check the pressure and temperature of each ball just before kickoff.
- Before handing a different ball to a team, check its temperature.
- If a temperature change of more than 5 °F occurs, check ball pressures. Adjust pressure if needed.
- If a number of balls are out of specification, check their temperatures. Adjust pressure if needed.
- Keep the footballs not in use out of the sun and the rain.
- Be aware that wind on a wet ball may lower its temperature and pressure even more.